Contact Info
Infectious Disease Epidemiology, Prevention and Control Division
651-201-5414
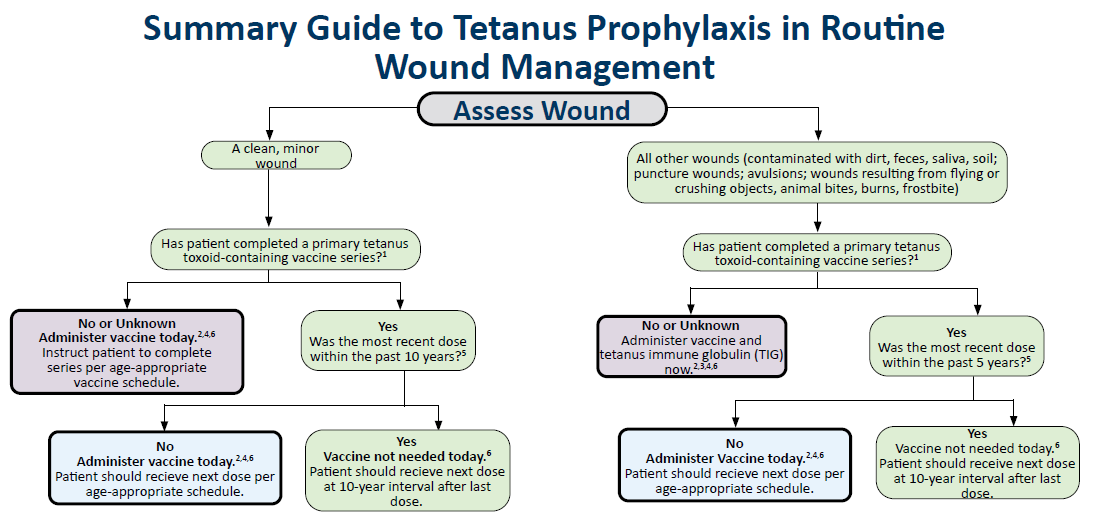
Summary Guide to Tetanus Prophylaxis in Routine Wound Management
A summary guide to examining wounds for possible tetanus prophylaxis.
PDF version formatted for print: Summary Guide to Tetanus Prophylaxis in Routine Wound Management (PDF)
- Primary series: A minimum of three doses of tetanus toxoid-containing vaccine (Tdap, DTaP or Td). Persons with unknown or uncertain tetanus vaccination history should be considered not vaccinated.
- Age-appropriate vaccine: Infants or children under 7 years of age: DTaP (or Td if pertussis component is contraindicated).
- Persons 7 years of age and older: Td is acceptable for routine 10-year boosters or catch-up vaccination.
- Tdap is preferred as the first dose for those 11 years of age and older who have not previously received Tdap.
- If Tdap has been given previously and another tetanus toxoid-containing dose is indicated (nonpregnant), either Td or Tdap may be used.
- Pregnancy: If a tetanus toxoid-containing dose is indicated, Tdap should be used.
- Persons 7 years of age and older: Td is acceptable for routine 10-year boosters or catch-up vaccination.
- Tetanus immune globulin (TIG):
- Dose: 250 U IM for all ages when indicated.
- Administration: If both TIG and vaccine are required, give simultaneously in separate syringes and at different anatomical sites.
- Indications (give TIG in the following situations):
- Persons with less than three documented doses of tetanus toxoid–containing vaccine, or with unknown vaccination history, who present with contaminated wounds.
- Infants less than 6 weeks of age with contaminated wounds (no vaccine is licensed at this age; give TIG only).
- Infants over 6 weeks of age who have received only one prior DTaP dose less than 4 weeks earlier and present with a contaminated wound. Give TIG now for immediate protection and defer the second DTaP dose until 4 weeks or more after the first dose so it is valid and can contribute to long-term immunity.
- Persons with HIV infection or severe immunodeficiency who present with contaminated wounds; give TIG regardless of prior tetanus vaccination history.
- Already protected: Persons who have completed a primary series of tetanus toxoid-containing vaccine AND received one dose of tetanus toxoid-containing vaccine within the past five years, do not require vaccine or TIG for wound management.
- Timing:
- Clean or minor wounds: Booster dose of tetanus toxoid-containing vaccine if less than 10 years since last dose.
- All other wounds (contaminated): Booster dose of tetanus toxoid-containing vaccine if 5 years or more since last dose.
- Arthus reaction: Persons with a history of an Arthus reaction after a tetanus toxoid–containing vaccine should not receive another tetanus-toxoid–containing vaccine until more than 10 years after the most recent dose, regardless of wound type.
Last Updated: 09/17/2025