Respiratory Syncytial Virus (RSV)
Infectious Respiratory Illness
- Infectious Respiratory Illness Home
- Viral Respiratory Illness in Minnesota (Data & Statistics)
- For Health Professionals
Related Topics
Contact Info
Respiratory Syncytial Virus (RSV) for Health Professionals
On this page:
Reporting
Infection prevention and control
Immunization options
Resources for health care providers
Reporting
- Reporting Respiratory Syncytial Virus (RSV)
As of Oct. 1, 2023, laboratory-confirmed RSV in all hospitalized patients who are Minnesota residents should be reported to the Minnesota Department of Health (MDH). - LTC Facility Influenza and RSV Report Form 2024-2025
MDH collects reports of outbreaks of RSV and influenza among residents living in long term care facilities. Please follow this link for more information and to fill out our online reporting form.
Infection prevention and control
Information for health professionals, schools, and childcare providers on RSV epidemiology, infection prevention, and control measures.
- American Academy of Pediatrics Red Book: RSV
American Academy of Pediatrics (AAP) reference with information on clinical manifestations, epidemiology, infection prevention, and control measures. - Hennepin County infectious diseases in childcare settings and schools manual
Section 6 contains fact sheets for school and child care providers on diseases including RSV. Information on signs and symptoms, spread, exclusion, and prevention and control.
Immunization options
Minnesota Respiratory Syncytial Virus (RSV) Vaccination Guide (PDF)
Information for the 2025-26 respiratory season for providers who handle or administer RSV vaccine. This guide summarizes vaccination recommendations, gives tips on vaccine administration, storage, and handling, and provides additional resources.
CDC recommends RSV vaccination for all adults 75 years of age and older and for adults 50-74 who are at increased risk of severe RSV disease. Adults who could benefit from vaccination due to higher risk of severe disease caused by RSV are those with underlying medical conditions, frailty or advanced age, and residents in long-term care facilities. Eligible adults can get an RSV vaccine at any time, but the best time to get vaccinated is in late summer and early fall before RSV usually starts to spread in communities.
Vaccine resources for older adults
- CDC: Respiratory Syncytial Virus (RSV) Immunizations
- CDC: ACIP Recommendations: Respiratory Syncytial Virus (RSV) Vaccine
- CDC: Current VISs
- CDC: Clinical Overview of RSV
- CDC: Diagnostic Testing for RSV
- CDC: About V-safe
Providers are urged to promote V-safe to adults ages 60 years and older and pregnant people 16-49 years of age who are vaccinated with RSV and have a computer, tablet, or smartphone. V-safe is a vaccine safety monitoring system that allows a person who is vaccinated to share with the CDC how they are feeling after vaccination.
To prevent severe RSV disease in infants, either maternal RSV vaccination or infant immunization with RSV monoclonal antibody is recommended. Most infants will not need both.
Vaccination during pregnancy
- Pfizer Abrysvo is the only RSV vaccine recommended during pregnancy.
- One dose should be given during weeks 32 through 36 of pregnancy, administered September through January (to protect infants born October through March).
- No additional doses should be given in subsequent pregnancies; the infant should receive a monoclonal antibody instead.
Monoclonal antibody prophylaxis
- Nirsevimab (also known as Beyfortus) and clesrovimab (also known as Enflonsia) are a long-acting RSV monoclonal antibody recommended for infants/toddlers to prevent lower respiratory tract disease from RSV.
- Monoclonal antibodies should be given:
- In the first week of life for infants born shortly before and during the season.
- Shortly before the start of the RSV season for infants aged less than 8 months (October through March).
- For nirsevimab only: Shortly before the start of the RSV season for children aged 8 to 19 months who are at increased risk of severe RSV disease or are American Indian and Alaska Native.
- The dosage is dependent on the product used:
- Nirsevimab is weight dependent:
- Infants less than 5kg (11 pounds) get 50mg.
- Infants 5kg (11 pounds) and over get 100mg.
- Clesrovimab is 105mg for all infants regardless of weight.
- Nirsevimab is weight dependent:
- The supply of monoclonal antibody products is projected to be adequate for the 2025-2026 season. Clesrovimab is not currently available for ordering on the federal contract, so is not currently available for VFC eligible children. It is expected to be available later this year.
- For more information visit Healthcare Providers: RSV Immunization for Infant and Young Children.
Vaccine resources for infant and pregnant people
- CDC: ACIP Recommendations: Respiratory Syncytial Virus (RSV) Vaccine
- CDC: Current VISs
- CDC: Respiratory Syncytial Virus (RSV) Immunizations
- Respiratory Syncytial Virus (RSV) Preventive Antibody: Immunization Information Statement (IIS)
- CDC: Clinical Overview of RSV
- CDC: Diagnostic Testing for RSV
- AAP: Nirsevemab Administration Visual Guide (PDF)
- CDC About V-safe
Providers are urged to promote V-safe to adults ages 50 years and older and pregnant people 16-49 years of age who are vaccinated with RSV and have a computer, tablet, or smartphone. V-safe is a vaccine safety monitoring system that allows a person who is vaccinated to share with the CDC how they are feeling after vaccination.
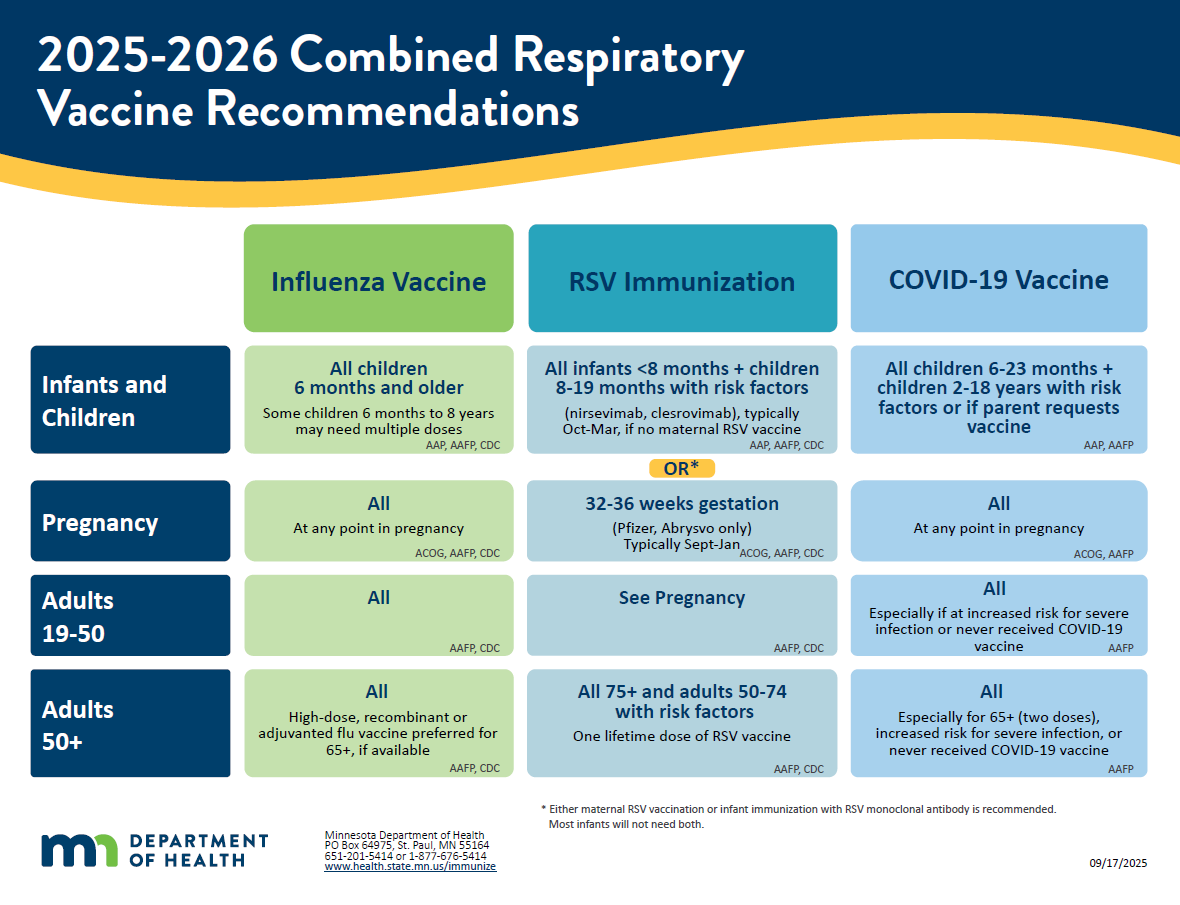
2025-2026 Combined Respiratory Vaccine Recommendations (PDF)
Handout version of the respiratory vaccine recommendations above.
Resources for health care providers
These resources can be used to explain and encourage vaccination and immunization for pregnant people and/or infants as well as help them keep record of it.
Communication between the pregnant person and infant providers will be important to ensure that all infants are protected.
 | Abrysvo: Respiratory Syncytial Virus (RSV) Vaccine Card (one card) (PDF)
|
 | RSV Immunization Card (one card) (PDF)
|
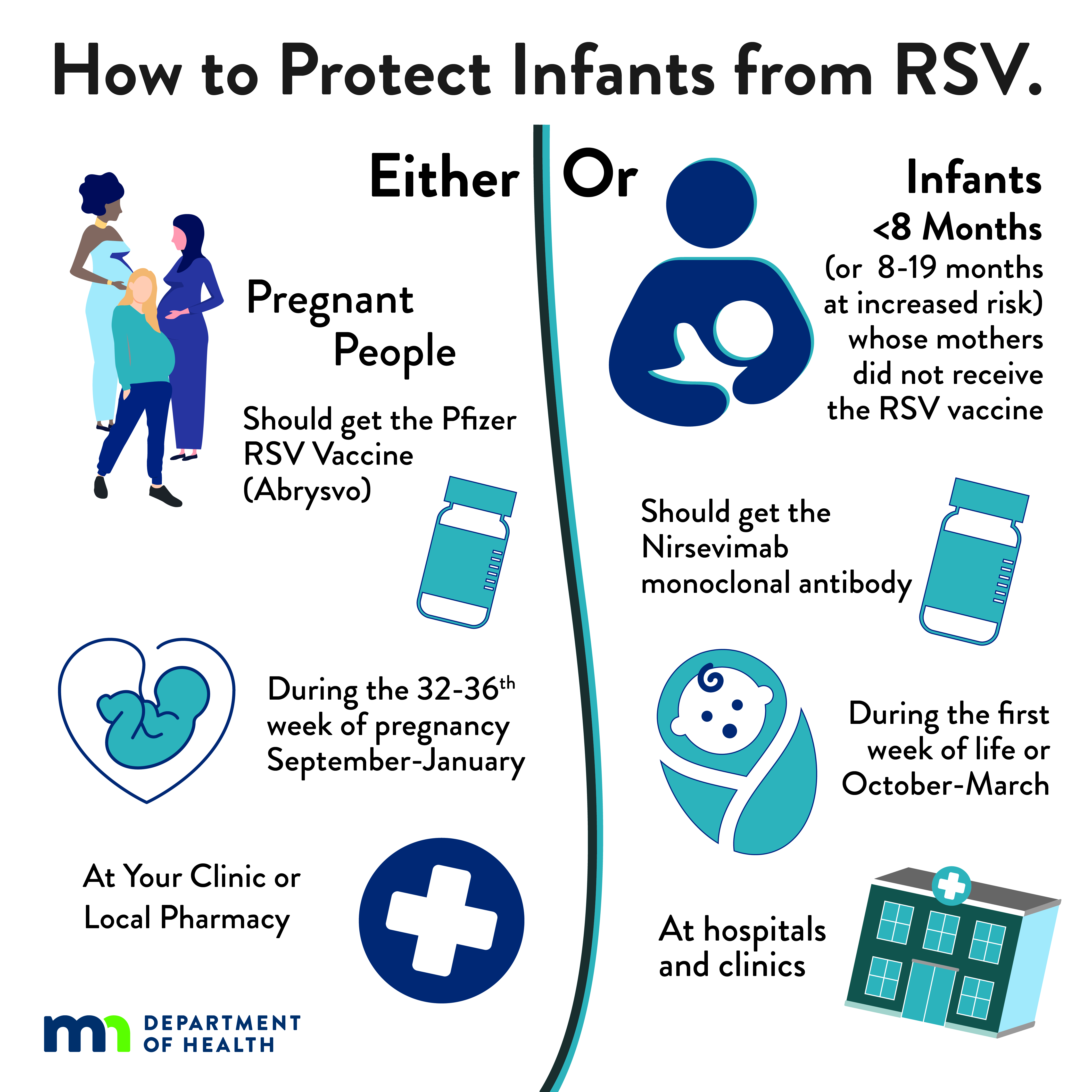 | Infographic: How to Protect Infants from RSV: Infants or Pregnant People (JPG) Social media infographic explaining both the RSV vaccine for pregnant people and the immunization for infants. |
