Contact Info
Infectious Disease Epidemiology, Prevention and Control Division
651-201-5414
Vaccine Storage Guide
Outdated or improperly stored vaccines won't protect patients!
Updated 1/19
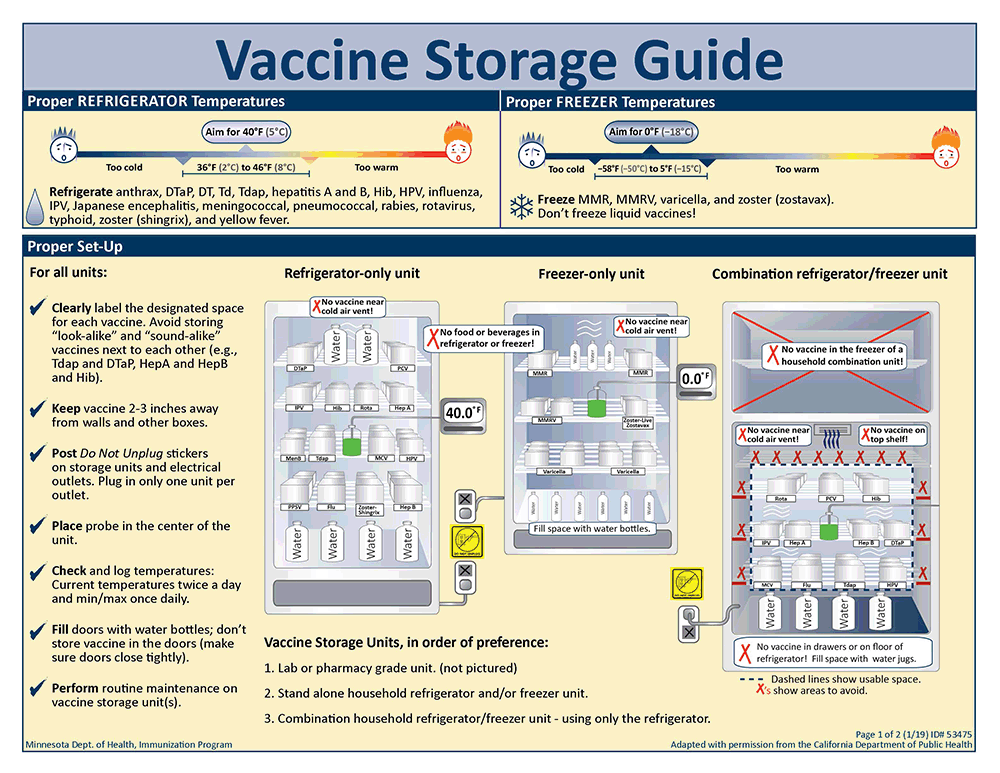
Vaccine Storage Guide: Two-page illustrated guide to vaccine storage temperatures and where and how to store vaccine in a refrigerator-only unit, freezer-only unit, and combination refrigerator/freezer unit. Includes tips for proper storage and management of vaccines.
Download PDF version formatted for print: Vaccine Storage Guide (PDF)
For all units
- Clearly label the designated space for each vaccine. Avoid storing "look-alike" and "sound-alike" vaccines next to each other (e.g., Tdap and DTaP, HepA and HepB and Hib).
- Keep vaccine 2-3 inches away from walls and other boxes.
- Post Do Not Unplug stickers on storage units and electrical outlets. Plug in only one unit per outlet.
- Place probe in the center of the unit.
- Check and log temperatures: Current temperatures twice a day and min/max once daily.
- Fill doors with water bottles; don't store vaccine in the doors (make sure doors close tightly).
- Perform routine maintenance on vaccine storage unit(s).
Vaccine storage units, in order of preference:
- Lab or pharmacy grade unit.
- Stand alone household refrigerator and/or freezer unit.
- Combination household refrigerator/freezer unit - using only the refrigerator.
Proper management
Designate one fully trained staff member to be the primary vaccine coordinator and at least one person to be backup. Ensure ongoing training for all immunization staff.
Manage vaccine inventory
- Review your vaccine inventory on a monthly basis and with each vaccine order to avoid over-ordering.
- If you have stock of both private and MnVFC vaccine, mark them clearly.
- Check vaccine expiration dates. Rotate your vaccine supply by placing vaccines with the earliest expiration dates in front of other vaccines and always use them first.
- Transfer vaccine you will not use before its expiration date to another MnVFC site to avoid wastage.
- Call the MnVFC program if you are unable to find another MnVFC site that can take your vaccine.
- Make sure you have enough space to store vaccine for the back-to-school rush and flu season.
Store vaccine correctly
- Place the temperature monitoring device’s probe in the center of the refrigerator or freezer with the vaccines.
- Use open trays, wire baskets, or other uncovered containers to help organize vaccines.
- Clearly label each container with the vaccine type. Avoid storing look-alike, sound-alike vaccines next to each other (e.g., Tdap and DTaP, HepA and HepB).
- Keep vaccines in their original packaging.
- Store vaccines in the middle of the unit, two to three inches from the walls, ceiling, floor, door, and cold air vent. Do not store vaccine in doors or drawers.
- Keep water bottles or jugs in the refrigerator and frozen water bottles in the freezer. Mark water bottles “DO NOT DRINK.”
- Routinely check that the door of each unit is shut.
Monitor temperatures
- Have a working calibrated continuous monitoring device (i.e., data logger) with a current and valid Certificate of Calibration in each unit that stores vaccine.
- Check and record current temperatures twice a day, at the start and end of each clinic day for all temperature monitoring devices including data loggers and continuous monitoring systems. Record the minimum and maximum temperatures at start of each clinic day. Reset device (if applicable) following manufacturer's instructions.
- Record temperatures on a temperature log and post it in a visible location or document electronically.
- Record the date, time, and name or initials of the individual checking the temperatures.
- Download temperature data and review weekly.
- Take immediate action on all out-of-range temperatures, including minimum and maximum temperatures!
- Keep temperature logs for three years.
Take action on out-of-range temperatures
Move vaccine immediately if refrigerated vaccine is less than 2°C (36°F).
- Determine the cause, if possible.
- Adjust the thermostat, if necessary.
- Notify your immunization manager or vaccine coordinator.
Monitor the temperature. If the temperature doesn’t stabilize in the correct range within 30 minutes:
- Stop using the vaccine.
- Mark the vaccine “DO NOT USE.”
- Move the vaccine to a storage unit that is maintaining the correct temperature.
- Collect the lot numbers, expiration dates, storage unit temperatures, the room temperature, and the time the unit was out-of-range.
- Evaluate the temperature data.
- Determine if any of this vaccine was involved in a previous mishap.
- Call the vaccine manufacturer(s).
- For MnVFC vaccine, call the MnVFC program at 651-201-5522 to report the mishap.
- Document your actions.
Last Updated: 10/26/2022