Health Risk Limits Rules
- Health Risk Limits Rules Home
- Rule Amendments
- Overview and Links
- Comments Received
- Contaminants
- Dual Notice
- Proposed Rules
- Request for Comments
- SONAR
- Current HRL Rules
- Rulemaking & Guidance History
- SONARs
Related Topics
Environmental Health Division
How Health-Based Values and Health Risk Limits are Calculated
The following is an excerpt from the 2008-2009 Statement of Need and Reasonableness (SONAR) (PDF) describing how health-based values (HBVs) are calculated. HBVs that are adopted into rule through the rulemaking process become Health Risk Limits (HRL). Therefore, all HRLs are calculated using the process described below. A Health-Based Water Guidance glossary (PDF) of terms is also available.
Introduction
An MDH-derived HRL is the concentration of a chemical in drinking water that, based on the current level of scientific understanding, is likely to pose little or no health risk to humans, including vulnerable subpopulations. This concentration is a function of how toxic a chemical is (that is, the minimum quantity that will cause health effects), the duration of exposure, and the amount of water individuals drink during the exposure period. In addition, a HRL value incorporates several adjustment factors to account for uncertainty in our understanding of a chemical’s health risks; chemicals with fewer studies will tend to have a higher degree of conservatism built into the HRL value to compensate for the higher degree of uncertainty.
The accepted method for assessing potential toxicity to humans is through controlled laboratory studies using mammals. In toxicity testing, animals are divided into groups and each group is administered one of several doses of a chemical, usually daily, over a set period of time. Testing has two goals: first, to identify the hazard or toxic effects caused by the chemical; and second, to evaluate the relationship between the dose and the animal’s response. The dose-response relationship may vary depending on when (e.g., the life stage) and for how long (duration) the exposure occurred.
In evaluating the dose and the response, researchers seek to determine the lowest dose at which adverse effects related to dosing are observed (the “lowest observed adverse effect level,” or LOAEL) and the highest dose at which no adverse effects related to dosing are observed (the “no observed adverse effect level,” or NOAEL). Alternatively, researchers may statistically model the data to determine the dose expected to result in a response in a pre-determined percent of the dosed animals (e.g., the benchmark dose). The dose resulting from dose-response evaluation (also referred to as a point of departure (POD) dose) serves as the starting point for deriving health-protective concentrations for environmental media.
For noncancer effects, the dose selected from the dose-response evaluation is reduced by variability and uncertainty factors (UFs) to account for what is not known about a chemical’s toxicity to a human population.
The factors account for:
- uncertainty in extrapolating from animal data to humans;
- variation in sensitivity among human individuals;
- uncertainty in extrapolating from effects observed in a short-term study to potential effects from a longer exposure;
- uncertainty associated with using a study in which health effects were found at all doses tested; and
- deficiencies in available data.
In the absence of chemical-specific information, each of the five factors is typically assigned a value between 1 and 10. Values of 1 and 10 are most common, but other values, such as 100.5 (half of 10 on a logarithmic scale, or approximately 3) are sometimes used.
Values assigned to all factors are multiplied to determine the overall uncertainty factor. Half-power values (e.g., 100.5) are factored as whole numbers when they occur singly but as powers or logs when they occur in tandem. Therefore, individual UFs of 3 and 10 would be expressed as 30 (3 × 101), whereas individual UFs of 3 and 3 would be expressed as 10 (100.5 × 100.5 = 101). The product of multiplying uncertainty and variability factors is usually at least 100. If the uncertainty associated with a chemical’s toxicity warranted application of uncertainty and variability factors whose product exceeded 3,000, MDH deemed that it had insufficient chemical information to derive an RfD (and therefore a HRL). The dose level selected from the dose-response evaluation (i.e., the point of departure dose, POD) is divided by the product of the uncertainty and variability factors to calculate a reference dose (RfD). An RfD is expressed in milligrams of chemical per kilogram of body weight per day (mg/kg-day) and is defined as an estimate of a dose level that is likely to be without an appreciable risk of adverse effects.
Understanding the relationship between timing and duration of exposure and the subsequent adverse effect is essential in deriving criteria that are protective of sensitive life stages (e.g., development) and short periods of high exposure (e.g., infancy). EPA has recommended the derivation of acute, short-term, subchronic, and chronic RfDs. If sufficient toxicological information was available, MDH derived RfDs for the various duration periods defined by EPA. The RfD values derived would be protective of all types of adverse effects for a given duration of exposure.
In the HRL revision, MDH has listed not just the noncancer effects occurring at the lowest effect dose, but also effects that occur at similar doses, also called "co-critical effects.". This provides more information to risk managers and can affect the results of an assessment when multiple chemicals are present. MDH has also indicated which chemicals are associated with endocrine effects and which chemicals have their greatest effects as a result of exposure in utero or during child development. In documentation supporting the rules, MDH has noted whether the information that it reviewed for each chemical includes assessments of developmental, reproductive, immunological, endocrine, or neurological effects.
HRLs for most carcinogens employ the default assumption for linear carcinogens, i.e., that any amount of exposure, no matter how small, potentially carries some risk. Derivations of HRLs based on the endpoint of cancer for chemicals considered to be linear carcinogens do not, therefore, employ an RfD. Instead, Minnesota’s long-standing public health policy is to derive values that limit the excess cancer risk to 1 in 100,000. Cancer potency is expressed as an upper bound estimate of cases of cancer expected from a dose of one milligram of substance per kilogram of body weight per day (i.e., cancer incidence per 1 mg/kg-day). From these estimates, a cancer potency slope, or “slope factor” (SF), can be calculated.
In standard cancer assays, animals are dosed only during their adult lives; early life is not included in the dosing and assessment period. Differences among infants, children, adolescents, and adults in absorption, distribution, biotransformation, excretion, target organ sensitivity, cell-protective mechanisms, and homeostatic control suggest that cancer may develop and progress differently among these age groups. MDH evaluated recent data analyses by EPA and other researchers that examine whether the timing and duration of an animal’s exposure to a carcinogen make a difference in the development of cancer. Generally, results indicate that cancer incidence from short-term early-life exposure can be similar to that from chronic adult-only exposure, and can be disproportionate to the duration of the exposure. In the 1993/1994 promulgations these analyses were not available.
The Groundwater Protection Act requires that MDH use cancer potency slopes published by EPA when deriving cancer HRLs. In calculating cancer HRLs in this revision, MDH used methodology contained in the recent EPA Supplemental Guidance for Assessing Susceptibility from Early-Life Exposure to Carcinogens to account for the potential for increased cancer potency when exposure occurs early in life. This approach involves applying age-dependent cancer potency adjustment factors to three life stages. The adjustment factors and corresponding life stages are: a 10-fold adjustment for individuals from birth to 2 years of age; a 3-fold adjustment for individuals from 2 to 16 years of age and no adjustment for individuals 16 years of age and older.
EPA has recommended that risk assessors apply this supplemental approach only to carcinogens with a mutagenic mode of action. In contrast, and based on comments from the Science Advisory Board and the external Expert Advisory Panel MDH adopted the EPA approach as a default approach for all linear carcinogens, regardless of whether the mode of action was known to be mutagenic. Chemical-specific information regarding life-stage sensitivity was used in place of the default approach whenever possible. Non-linear carcinogens, i.e., those for which cancer risk is not directly proportional to dose at low dose levels, were evaluated using an RfD approach as recommended in the recent EPA Final Guidelines for Carcinogenic Risk Assessment.
In the 1993/1994 HRL promulgations, MDH evaluated chemicals considered possible human carcinogens (EPA Group C classification) for noncancer effects and assigned an additional 10-fold uncertainty factor (a “Group C factor”) for possible carcinogenicity. In this revision, as recommended by the external Expert Advisory Panel, MDH conducted a case-by-case evaluation for such chemicals and used scientific judgment to determine whether or not the data supported a finding of carcinogenicity. If adequate evidence of carcinogenicity existed and an EPA cancer potency slope was available, a cancer HRL was derived. In the absence of an EPA cancer potency slope factor, application of a Group C factor was considered. If the evidence of carcinogenicity was inadequate, MDH derived a noncancer chronic HRL using a chronic RfD. In this case the noncancer HRL did not incorporate a Group C factor.
For HRL chemicals with both cancer and noncancer endpoints, MDH derived cancer and noncancer HRLs if toxicity data were sufficient.
In deriving HRLs, the RfD (for noncancer) or dose associated with additional cancer risk equal to or less than 1/100,000 (for cancer) is converted from mg/kg-day to a water concentration in micrograms per liter (μg/L) by dividing by an intake rate. Intake rate is expressed as the quantity of water consumed per kilogram of body weight per day (L/kg-day).
Studies of water consumption indicate that infants and young children drink more water for their body weight than do adults. The algorithm used for the 1993/1994 HRLs followed standard risk assessment practice at the time and used a default adult daily intake rate of two liters and a default adult body weight of 70 kilograms (equivalent to approximately 0.029 L/kg-day). Based on current intake information, 0.029 L/kg-day corresponds to the 86th percentile of adult consumers of water from community supplies.
Newborns derive all, or nearly all, their nutrition from liquid. Intake rates fall rapidly with age; by age seven, intake rates are nearly the same as those of adults. Generally, HRLs are thought of as protecting against adverse health effects from long-term exposures to contaminants in drinking water. However, they must protect against adverse effects from shorter exposures as well. MDH considered sensitive life stages and subpopulations as well as the magnitude and duration of exposure necessary to elicit a toxic effect.
EPA has recommended the evaluation of multiple exposure durations, including: acute – dosing up to 24 hours; short-term– repeated dosing for more than 1 day, up to approximately 30 days; subchronic– repeated dosing for more than 30 days, up to approximately ten percent of a lifespan in humans (more than 30 days up to approximately 90 days in typical laboratory rodent studies); and chronic– repeated dosing for more than approximately ten percent of a life span. The external Expert Advisory Panel also recommended that MDH evaluate less-than-chronic exposure durations to ensure that shorter periods of exposure were adequately protected. In this rules revision MDH has used a life expectancy of 78 years.
The relevant duration is defined from the time point in the assessment at which the adverse effect was first observed. Protocols for toxicity testing do not necessarily evaluate or report effects observed at interim time points (i.e., before the end of the study). The effects reported at the end of the study could have arisen earlier and thus may have resulted from a shorter duration. MDH acknowledges this limitation and the potential to overestimate the effective duration. However, in the absence of interim time point assessment information, MDH has opted to use the duration of the study as the relevant dosing duration.
MDH used data reported in EPA’s Per Capita Report and a revised assessment for the draft Child-Specific Exposure Factors Handbook to calculate default water intake rates for the various durations specified above in the past. Beginning in 2016, MDH began using EPA's finalized water intake rates in the 2011 Exposure Factors Handbook, as follows: acute or short term: 0.285 L/kg-day, based on the 95th percentile intake from 1 up to 3 months of age; subchronic: 0.070 L/kg-day, based on a time-weighted average (TWA) of the 95th percentile intake from birth up to 8 years of age; and chronic: 0.044 L/kg-day, based on TWA of the 95th percentile intake over a lifetime of approximately 70 years of age.
In addition to duration considerations MDH considered life-stage sensitivity. When the developmental period was identified as the most susceptible life stage, MDH selected the acute and short-term default intake rate for infants aged 1 up to 3 months (i.e., 0.285 L/kg-day) as the default intake for deriving HRLs based on developmental effects.
MDH has adopted EPA’s approach for integrating age-dependent sensitivity adjustment factors and exposure information for the derivation of HRLs for linear carcinogens. The default intake rates corresponding to the age-dependent adjustment factor age groups used in deriving cancer HRLs are based on the TWA of the 95th percentile intake rate for each age range. As of 2016, MDH is using the values of 0.125 L/kg-day (up to 2 years of age), 0.045 L/kg-day (2 to up to 16 years of age), and 0.041 L/kg-day (16 years of age and older). The lifetime duration used by EPA to characterize lifetime cancer risk is 70 years. Although this is no longer the life expectancy of the U.S. population, a value of 70 years corresponds to the equivalent duration over which health effects are typically assessed in chronic studies of laboratory animals. Therefore, 70 years has remained the standard definition of “lifetime” even as human life expectancy has increased.
MDH will depart from the above default intake rates if sufficient chemical-specific information indicates that a different duration or intake rate is more appropriate. In these cases MDH will calculate an appropriate TWA intake rate for the duration specified by the chemical-specific information.
An RfD or a cancer potency slope incorporates information about the toxicity of a single chemical associated with a given oral dose. Neither of these values, however, provides information about multiple exposures, whether from other routes of exposure (e.g., inhalation) or from multiple chemicals. Issues such as these are addressed in a risk assessment process called “risk characterization.”
The Groundwater Protection Act requires that MDH use a “relative source contribution” (RSC) factor when deriving HRLs for noncancer effects. The RSC allocates only a portion of the RfD to exposure from ingestion of water, and reserves the remainder of the RfD for other water-related exposures (e.g., inhalation of volatilized chemicals, dermal absorption) as well as exposures via other contaminated media such as food, air, and soil. MDH has relied upon EPA’s Exposure Decision Tree approach to determine appropriate default RSC values.
MDH derived HRLs for contaminants that are present in Minnesota’s groundwater solely as the result of human activity, or for naturally occurring compounds present at elevated levels due to human activity. HRLs are often used to make decisions for cleaning up pollutants at contaminated sites where media other than groundwater may also be contaminated. The level of media contamination, the completed routes of exposure and the potentially exposed populations will vary from site to site and from chemical to chemical. Using the Exposure Decision Tree, MDH determined the following default RSC values. For acute and short-term exposure (based on infant intake rates), the RSC is 0.2 for highly volatile contaminants or 0.5 for other chemicals; for subchronic or chronic exposures, the RSC is 0.2 for all chemicals. MDH recognizes that departures from the outlined approach may be appropriate in the application of HRLs by MDH or other state agencies, and there may be situations where the Exposure Decision Tree could be used in conjunction with site-specific information to derive a site-specific RSC. Such site-specific RSCs are not appropriate for general use as a statewide or regional value.
In response to requests made by state risk managers, MDH provided additional information about the toxicity of HRL chemicals and about strategies for more complex risk evaluations. The types of information, such as a more extensive list of noncancer health endpoints and both cancer and noncancer HRLs for a single chemical, have been noted above.
Certain chemicals dissolved in drinking water have a tendency to volatilize, or escape, into the air. For volatile chemicals, inhalation exposure may be a special risk management concern. The revised rules include a volatility classification (e.g., high, moderate, low or non-volatile) for each chemical. MDH included this so that risk managers could more readily conduct a site-specific evaluation of inhalation exposure in situations where highly volatile chemicals have contaminated the groundwater and this groundwater is used for domestic purposes (e.g., bathing, showering, etc.).
While toxicity is usually evaluated for individual chemicals, real-life exposures involve multiple chemicals. In the rules, MDH includes methods that risk managers can use to sum up the risks from multiple chemicals that share a common health endpoint in order to assess the combined health risk at the site being evaluated. This common health risk index approach is a default approach; if specific data about a mixture are available, other approaches may be used and, in fact, are likely to be preferable.
MDH Algorithms
MDH applied the various duration-specific intake rates to the default HRL algorithm for noncancer effects (nHRL): 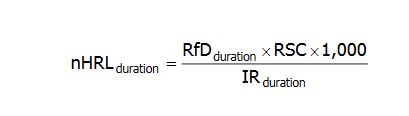
Where
nHRLduration = the noncancer health risk limit, for a given duration, expressed in units of micrograms of chemical per liter of water (&956;g/L).
RfDduration= the reference dose, for a given duration, expressed in units of milligram per kilogram per day (mg/kg-day). The following default durations are used: (i) acute – a period of 24 hours or less; (ii) short-term – a period of more than 24 hours, up to 30 days; (iii) subchronic – a period of more than 30 days, up to approximately 10% of the life span in humans; or (iv) chronic – a period of more than approximately 10% of the life span in humans.
RSC = the relative source contribution factor which represents the percentage of totalexposure to a substance or chemical that is allocated to ingestion of water. The default RSC is 0.2 for highly volatile chemicals. For other chemicals the default RSC is 0.5 for acute and short-term HRLs and 0.2 for subchronic or chronic HRLs.
1,000 = a factor used to convert milligrams (mg) to micrograms (&956;g).
IRduration = the intake rate of ingestion of water, or simply the amount of water, on a perbody weight basis, ingested on a daily basis (liters per kg body weight per day or L/kg-day). The default IR corresponds to the time-weighted average (TWA) of the 95th percentile intake rate during the relevant duration: acute and short-term - 0.285 L/kg-day, based on intake for 1 up to 3 months of age; subchronic - 0.070 L/kg-day, based on a TWA up to 8 years of age; and chronic - 0.044 L/kg-day, based on a TWA over a lifetime of approximately 70 years.
MDH will depart from the above default HRL algorithm and parameter values if sufficient chemical-specific information indicates that a different duration or intake r ate is more appropriate. In these cases a time-weighted intake rate would be calculated over the duration specified by the chemical-specific information. The RfD, RSC and IR values used for each chemical in deriving each nHRL are identified in the rules.
The magnitude of the HRL value is a function of the reference dose (RfD) and the intake rate. In general, for a given chemical, the shorter-duration RfD values will be higher than longer-duration RfD values because the human body can usually tolerate a higher dose when the duration of the dose is short, even if that same dose would be harmful when it occurs over a longer duration. In most cases, therefore, the calculated HRL values decrease with increasing duration, e.g., acute HRLs are greater than short-term HRLs, short-term HRLs are greater than subchronic HRLs, and so on. It is possible, however, that the RfD for a shorter-duration is the same, or in rare cases lower, than the RfD for a longer-duration. This could result if a short-duration was sufficient to elicit an adverse effect, if a more sensitive endpoint was assessed in the shorter-duration study, or if a different species or life stage was assessed. The intake rate also impacts the magnitude of the HRL value. As shown above, the shorter duration intake rates are higher than the longer-term intake rates. These factors may cause a calculated shorter-duration HRL to be less (lower) than a longer-duration HRL; when this occurs, the longer-duration HRL is set equal to the lower, shorter-duration HRL. This ensures that the HRL for a longer duration is protective of any higher shorter-term exposure that occurs within its defined time span. For example, it would not be prudent to promulgate a short-term HRL of 20 μg/L and a subchronic HRL of 80 μg/L even if analysis of the available studies and intake rates yielded these values. The subchronic value implies that 80 μg/L has no adverse effects for an exposure duration of 30 days up to 10% of a lifetime, but the short-term study has established that 20 μg/L is the appropriate limit for shorter exposures (30 days). Adoption of 80 μg/L for the subchronic HRL would allow exposures that exceed the 20 μg/L short-term HRL. In this instance, logic dictates that the 80 μg/L subchronic value be overridden by the short-term value of 20 μg/L. When a substitution occurs, the table of HRL values and endpoints may list endpoints for the longer-duration HRL that are not applicable to the substituted value when it is used for shorter durations. This is done to assure that any potential relevant health endpoints are not discarded when the substitution is made.
For the derivation of cancer HRLs for linear carcinogens, MDH applied EPA’s age-dependent cancer potency adjustment factors and corresponding intake rates to the default HRL algorithm for cancer: 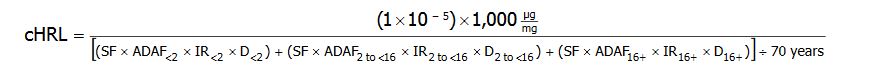
Where
cHRL = the cancer health risk limit expressed in units of micrograms of chemical per liter of water (&956;g/L).
(1×10-5) = the additional cancer risk level.
1,000 = a factor used to convert milligrams (mg) to micrograms (μg).
SF = the cancer slope factor for adult exposure, expressed in units of the inverse of milligrams per kilogram of body weight per day ([cancer incidence per mg/kg-day] or [mg/kg-day]-1)
ADAF = the age-dependent adjustment factor for each age group: 10, for up to 2 years of age (ADAF<2); 3, for 2 up to 16 years of age (ADAF2 to <16); and 1, for 16 years of age and older (ADAF16+).
IR = the intake rate for each age group: 0.137 L/kg-day, for up to 2 years of age (IR<2); 0.047 L/kg-day, for 2 up to 16 years of age (IR2 to <16); and 0.039 L/kg-day, for 16 years of age and older (IR16+).
D = the duration for each age group: 2 years, for up to 2 years of age (D<2); 14 years, for 2 up to 16 years of age (D2 to <16); and 54, for 16 years of age and older (D16+).
70 years = the standard lifetime duration used by EPA in the characterization of lifetime cancer risk.
MDH departed from the above default HRL algorithm if sufficient information was available to derive a chemical-specific lifetime adjustment factor (AF lifetime). In these cases MDH applied a time-weighted intake rate over a lifetime, resulting in the following equation:
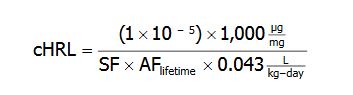
Where
(1×10-5) = the additional cancer risk level
1,000 = a factor used to convert milligrams (mg) to micrograms (microgram)
SF = adult-exposure based cancer slope factor
AF lifetime = the lifetime adjustment factor based on chemical-specific data
0.043 L/kg-day = 95th percentile water intake rate representative of a lifetime period
The SF, slope factor adjustment, and IR values used for each chemical in deriving each cHRL will be identified in the rules. Adjustments to the toxicity and intake rate components of the risk algorithm, along with MDH’s evaluation of less-than-chronic durations and developmental and reproductive testing, are the foundation of MDH’s efforts to ensure that HRLs protect all life stages.
The RfD and the cancer slope factor use scientifically sound methods to analyze the most current available toxicological data while continuing to make use of preexisting data.
In accordance with the general rule for calculations involving multiplication or division, HRLs are rounded to the same number of significant figures as the least precise parameter used in their calculation. As a result, the HRL values were typically rounded to one significant figure. Rounding was performed as the final step in the calculation process.
The following rounding procedures were used: (1) if the digit 5, 6, 7, 8, or 9 is dropped, increase the preceding digit by one unit; (2) if the digit 0, 1, 2, 3, or 4 is dropped, do not alter the preceding digit.
