Contact Info
Tuberculin Skin Test (TST)
Tuberculin skin tests (TST) are administered to detect the presence of Mycobacterium tuberculosis, the bacterium that causes tuberculosis (TB).
The terms Mantoux, TB skin test, tuberculin skin test, and PPDs are often used interchangeably. Mantoux refers to the technique for administering the test. Tuberculin (also called purified protein derivative or PPD) is the solution used to administer the test. The preferred term for the test is tuberculin skin test, or TST.
People who have been vaccinated with Bacille Calmette-Guérin (BCG) should not be exempted from TB skin testing unless they have a documented positive result from a prior test. Disregard BCG history when interpreting TST results.
On this page:
TST Basics
TST Documentation Requirements
Two-Step TSTs
BCG
Adverse Reactions to TSTs
Training Resources
TST Basics
- Tuberculin Skin Testing (TST): Storage of Tuberculin and Need for Orders to Administer (PDF)
- Tuberculin Skin Testing (TST) Protocol for Screening Health Care Workers (Word)
Template protocol developed by MDH. Includes guidance for administration, reading, and interpreting TSTs. Note: to remove the watermark on the document, click on the document's footer then click on the watermark text.
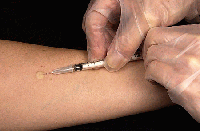 Injecting tuberculin; Source: Centers for Disease Control and Prevention
Injecting tuberculin; Source: Centers for Disease Control and Prevention
- The TST is an intradermal injection of 0.1 ml of tuberculin (PPD) on the inner surface of the forearm.
- The skin test reaction should be read between 48 and 72 hours after administration.
- If the test is not read within 72 hours, another TST should be placed unless the amount of induration is ≥ 10 mm within 7 days after placement.
- The reaction should be measured in millimeters of induration (palpable, raised, hardened area or swelling).
- Do not measure erythema (redness).
- The indurated area should be measured across the forearm (perpendicular to the long axis).
- Health care workers (HCWs), patients, or their family members should not be allowed to record their own TST results.
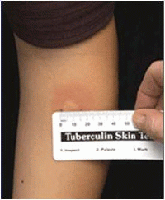 Measuring induration; Source: Centers for Disease Control and Prevention
Measuring induration; Source: Centers for Disease Control and Prevention
- Candidates for Treatment of Latent Tuberculosis Infection (PDF)
The cut-off points for a positive TST result depend on the individual’s risk factors for TB exposure or for developing active TB disease if infected.
- People who have been vaccinated with BCG should not be exempted from TB skin testing unless they have a documented positive result from a prior test. Disregard BCG history when interpreting TST results.
- HCWs and patients with positive TST results must receive appropriate medical follow up.
TST Documentation Requirements
At time of administration:
- Name and signature of person administering test
- Date and time test administered
- Location of test (e.g., right forearm, left forearm, alternate site)
- Tuberculin manufacturer, lot number and expiration date
At time of reading:
- Name and signature of person reading test
- Date and time test read
- Exact number of mm of induration (if no induration, document "0" mm)
- Interpretation of reading (i.e., positive or negative, based on individual's risk factors)
Two-Step TSTs
- A two-step TST is required for baseline TB screening of HCWs and patients in boarding care facilities, correctional facilities, and nursing homes.
- Two-step TSTs are not recommended for patients in other settings.
- A two-step TST is performed at baseline because people who were infected with TB many years ago may have a negative reaction to an initial TST.
- The first "step" may stimulate (or boost) the immune system's ability to react to the test.
- If the second "step" is not performed as part of baseline screening, a subsequent positive TST reaction could be misinterpreted as a new infection.
- Follow the directions in the Indications for two-step tuberculin skin tests (TPDF) chart provided by the Centers for Disease Control and Prevention (CDC) to perform two-step TSTs.
BCG
- BCG, or bacille Calmette-Guérin, is a vaccine for TB.
- BCG is used in many countries with a high prevalence of TB to prevent childhood tuberculous meningitis and miliary disease.
- However, BCG is not generally recommended for use in the United States because of the low risk of infection with Mycobacterium tuberculosis, the variable effectiveness of the vaccine against adult pulmonary TB, and the vaccine's potential interference with tuberculin skin test reactivity.
- TSTs and TB blood tests to detect TB infection are not contraindicated for persons who have been vaccinated with BCG.
- Evaluation of TST reactions in persons vaccinated with BCG should be interpreted using the same criteria for those not BCG-vaccinated.
- Unlike the TST, TB blood tests do not detect the presence of BCG and are less likely to give a false-positive result.
Adverse reactions to TSTs
- Severe adverse reactions to TSTs are considered rare.
- Severe reactions include anaphylaxis (extremely rare), severe swelling, heavy blistering, or "weeping" of the skin.
- If an individual does not have documentation of a severe adverse reaction but provides a convincing verbal report, document the severe adverse reaction and do NOT administer another TST.
- Substitute a TB blood test (IGRA) for the TST if it is available in your area.
- Mild itching, swelling, or irritation are more common and are not a contraindication to future testing.
- Individuals should be instructed to avoid scratching or scrubbing the site, to keep the site clean and dry, and to avoid putting lotions or adhesive bandages on it.
- A cold compress may be helpful.
Training Resources
- CDC: Bacille Calmette-Guérin (BCG) Vaccine for Tuberculosis
Fact sheet from the CDC that presents the basics about BCG. - CDC: Mantoux Tuberculin Skin Test Toolkit
Testing DVD, facilitator guide, ruler, and wall chart provided by CDC for download or order. - CDC: Mantoux Tuberculin Skin Testing Wall Chart
Free 24" x 28" poster from CDC that presents TST administration, reading, and interpretation. - CDC: Tuberculin Skin Testing Fact Sheet;
CDC fact sheet that discusses TST administration, reading, and interpretation.
