Lead
- Lead Home
- Common Sources of Lead
- Contractor Information
- Educational Materials
- Factsheets, Videos and Brochures
- Lead Contractor Search
- Lead Individual License Search
- Lead Licensing
- Health Care Professionals
- Home Owner Information
- Laws and Rules
- Rule Revision: Lead Laws and Rules
- Lead in Schools
- Lead Resources
- Lead Reports
- M-CLEAN
- File a Complaint Related to Lead
- Lead Contacts
Related Sites
- Birth Defects Monitoring and Analysis
- Children's Environmental Health
- DWP Fact Sheets
- Individual and Family Health
- Lead in Well Water
- Nutrition: Healthy Eating
Environmental Health Division
Blood Lead Electronic Data Submission
Test result reporting
Minnesota Statutes, section 144.9502, subd. 3, requires laboratories to submit all blood lead test results to the Minnesota Department of Health (MDH). This includes tests conducted on point-of-care machines.
For programs that perform more than 50 blood lead tests per year, MDH will no longer be accepting mailed or faxed results. At a minimum, results may be submitted using a spreadsheet. For setting up blood lead test result reporting via spreadsheet, contact MDH Lead Surveillance Database Coordinator at Health.bloodleadresults@state.mn.us or (651) 201-4919.
Programs that perform 50 or less blood lead tests per year are encouraged to submit blood lead test results electronically, but may submit results by mail or fax. Reporting Blood Lead Test Results has more information on reporting blood lead data, including Blood Lead Test Result report form for manual data submission.
MDH strongly encourages facilities to submit data using electronic lab reporting with HL7 messages. The process for setting up HL7 messages is outlined below under Blood Lead Electronic Data Submission: HL7 Messages.
Blood lead electronic data submission: HL7 Messages
Blood Lead Reporting is a public health specialized registry for meaningful use or MIPS (MACRA) in Minnesota. This specialized registry option is available to eligible professionals/eligible clinicians who conduct blood lead testing, including tests using point-of-care machines, and report results electronically to MDH using the HL7 2.5.1 ELR specification. Priority for onboarding will be given to providers who submit results from point-of-care machines. Reporting lead results from hospitals is covered under the ELR (electronic lab reporting) option for meaningful use.
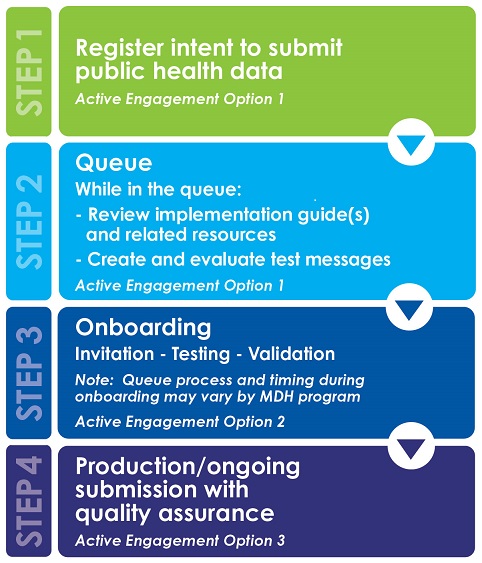
Pre-testing
Sending information to MDH electronically does not satisfy the meaningful use/MIPS (MACRA) criteria by itself. The information must be sent using standards that have been developed to send health-related information between health care information systems. As part of pre-testing, the MDH blood lead program requires all facilities/laboratories to generate and evaluate test messages prior to submitting test messages to MDH. MDH will provide message formatting information prior to the pre-testing process.
Onboarding
Onboarding includes testing and validation and then moving to production/ongoing submission. A queue may be initiated depending on the number of clinics/clinicians/professionals registered and the priority list established by the MDH Blood Lead Program. Priority for onboarding will be given to providers who submit results from point-of-care machines.
Questions
Contact the MDH Lead Surveillance Database Coordinator at health.bloodleadresults@state.mn.us or (651) 201-4919 for questions and more information.
